› Forums › General Melanoma Community › Ipi and radiation–a couple of details
- This topic has 6 replies, 2 voices, and was last updated 13 years, 11 months ago by
jim Breitfeller.
- Post
-
- March 8, 2012 at 7:36 pm
Last night's report on ipi and radiation set of a lot of activity, as seen from several threads below. One person from the MPIP community had some specific questions and I was able to get some answers. I thought this might be of interest to others here:
Last night's report on ipi and radiation set of a lot of activity, as seen from several threads below. One person from the MPIP community had some specific questions and I was able to get some answers. I thought this might be of interest to others here:
1. How far out was the patient from the last dose of the ipi regimen? (And was she on the standard approved protocol or some other dose/frequency program?) The patient was on a trial in which maintenance ipi was given every 12 weeks at 10 mg/kg. She was between 2 such maintenance doses when the radiation was done.
2. What was the size of the tumor that was irradiated?About 5 cm.
3. Any reason to think that radiation of several smaller tumors might have a similar effect? It certainly could.
I think it is important to remember that this is the story of one patient. No-one knows yet if what happened with her is indicative of what will happen to a braoder group. Having said that, the principle behind the report is consistent with other immunologic approaches. A handful of companies are working on vaccines that involve tumor specific antigens, based on the same concept that these tumor proteins can stimulate an immune response against the tumor as a whole. Some of those companies are already discussing doing trials combining their vaccine with ipi.
I get frustrated with media expanding a small positive result into a world changing event. It may be such an event, but it may not be. In the meantime I am quite sure that doctors across the country are being approached by ipi patients asking for radiation….
Hopefully this is a true turning point. Dr. Wolchok does good work, and Sloan Kettering has an excellent program, so it comes from a reputable source.
Tim–MRF
- Replies
-
-
- March 8, 2012 at 10:05 pm
I just came from my drs office and asked him about this report. He said it can take 7 years of research and testing before it ever gets proven and to get to the media. He has Known about it for some time now and has given radiation where needed. He said it does have good results. -
- March 8, 2012 at 10:05 pm
I just came from my drs office and asked him about this report. He said it can take 7 years of research and testing before it ever gets proven and to get to the media. He has Known about it for some time now and has given radiation where needed. He said it does have good results. -
- March 8, 2012 at 10:05 pm
I just came from my drs office and asked him about this report. He said it can take 7 years of research and testing before it ever gets proven and to get to the media. He has Known about it for some time now and has given radiation where needed. He said it does have good results. -
- March 8, 2012 at 11:08 pm
Tim,
This Radiation has been known for quite some time. Even Dr. Rosenberg is using whole body irradiation before Adoptive Cell Therapy.
I myself created a diagram of what may be taking place in vivo.
It has been known that if radiation kills the outer most cells of the tumor, it can shed tumor specific peptides/antigens that are picked up and display on the DCs. It can also get the cells to secrete HMGB-1 which binds to the TLR4 and activates the macrophages which in turn secrete IL-12 and other cytokines and chemoattractants to get the right immune response.
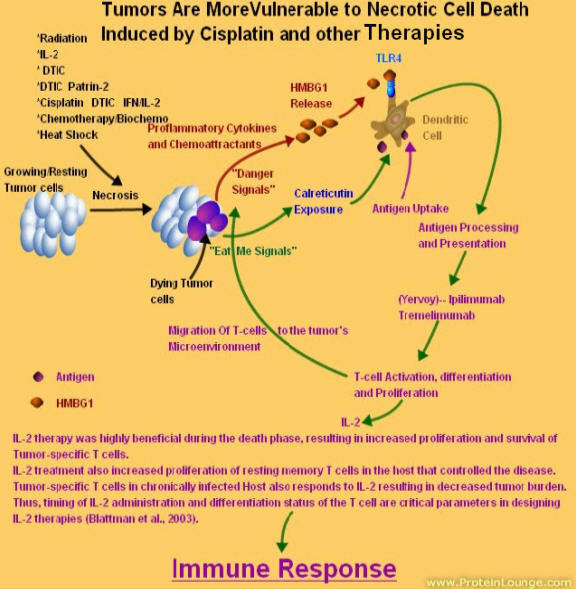
At least part of the inflammatory effect of HMGB1 may be due to its ability to induce IL-1 production by monocytes in a CD14 and TLR4-dependent manner, resulting in production of IFN-γ and IL-17 by T cells.
Furthermore, HMGB1 signaling via TLRs activates the transcription factor NF-κB, which itself induces further upregulation of HMGB1 and its receptors thereby amplifying inflammation in a positive feedback loop.
Dr. Rosenberg uses whole body radiation prior to the adoptive cell therapy. He has seen higher responses.
The Immune System needs three Signals:
The role of the CD8+ T cells is to monitor all the cells of the body, ready to destroy any that express foreign antigen fragments in their class I molecules.
Three major events must occur to Activate CD8+ T cell mediated response against melanoma.
First, the T-cell receptor (TRC) must be triggered by a (or multiple) self antigen–derived peptide MHC class I complex12. This event depends entirely on appropriate antigen presentation, which is most efficiently provided by mature dendritic cells. Once properly activated, may serve as tumor-specific effector T cells.
Second, simultaneously with T-cell receptor triggering, a distinct second costimulatory signal must be delivered, mediated by IL-2, B7-1, or B7-2, which engage IL-2 receptors and CD28 on the surface of the T cell, respectively . A source of these cofactors for effective CD8+ T-cell stimulation can be provided by CD4+ T cells that release critical amounts of IL-2, or by mature dendritic cells that display an increased level of B7-1/B7-2 costimulatory molecules on their cell surfaces.
Third, inflammatory cytokines, including IL-1, IL-6, IL-12, and IFN-γ provide a third signal that acts directly on T cells, referred to as the “danger signal”. This signal was found to optimally activate TH1 differentiation and lead to clonal expansion of T cells.
Best Regards,
Jimmy B
-
- March 8, 2012 at 11:08 pm
Tim,
This Radiation has been known for quite some time. Even Dr. Rosenberg is using whole body irradiation before Adoptive Cell Therapy.
I myself created a diagram of what may be taking place in vivo.
It has been known that if radiation kills the outer most cells of the tumor, it can shed tumor specific peptides/antigens that are picked up and display on the DCs. It can also get the cells to secrete HMGB-1 which binds to the TLR4 and activates the macrophages which in turn secrete IL-12 and other cytokines and chemoattractants to get the right immune response.

At least part of the inflammatory effect of HMGB1 may be due to its ability to induce IL-1 production by monocytes in a CD14 and TLR4-dependent manner, resulting in production of IFN-γ and IL-17 by T cells.
Furthermore, HMGB1 signaling via TLRs activates the transcription factor NF-κB, which itself induces further upregulation of HMGB1 and its receptors thereby amplifying inflammation in a positive feedback loop.
Dr. Rosenberg uses whole body radiation prior to the adoptive cell therapy. He has seen higher responses.
The Immune System needs three Signals:
The role of the CD8+ T cells is to monitor all the cells of the body, ready to destroy any that express foreign antigen fragments in their class I molecules.
Three major events must occur to Activate CD8+ T cell mediated response against melanoma.
First, the T-cell receptor (TRC) must be triggered by a (or multiple) self antigen–derived peptide MHC class I complex12. This event depends entirely on appropriate antigen presentation, which is most efficiently provided by mature dendritic cells. Once properly activated, may serve as tumor-specific effector T cells.
Second, simultaneously with T-cell receptor triggering, a distinct second costimulatory signal must be delivered, mediated by IL-2, B7-1, or B7-2, which engage IL-2 receptors and CD28 on the surface of the T cell, respectively . A source of these cofactors for effective CD8+ T-cell stimulation can be provided by CD4+ T cells that release critical amounts of IL-2, or by mature dendritic cells that display an increased level of B7-1/B7-2 costimulatory molecules on their cell surfaces.
Third, inflammatory cytokines, including IL-1, IL-6, IL-12, and IFN-γ provide a third signal that acts directly on T cells, referred to as the “danger signal”. This signal was found to optimally activate TH1 differentiation and lead to clonal expansion of T cells.
Best Regards,
Jimmy B
-
- March 8, 2012 at 11:08 pm
Tim,
This Radiation has been known for quite some time. Even Dr. Rosenberg is using whole body irradiation before Adoptive Cell Therapy.
I myself created a diagram of what may be taking place in vivo.
It has been known that if radiation kills the outer most cells of the tumor, it can shed tumor specific peptides/antigens that are picked up and display on the DCs. It can also get the cells to secrete HMGB-1 which binds to the TLR4 and activates the macrophages which in turn secrete IL-12 and other cytokines and chemoattractants to get the right immune response.

At least part of the inflammatory effect of HMGB1 may be due to its ability to induce IL-1 production by monocytes in a CD14 and TLR4-dependent manner, resulting in production of IFN-γ and IL-17 by T cells.
Furthermore, HMGB1 signaling via TLRs activates the transcription factor NF-κB, which itself induces further upregulation of HMGB1 and its receptors thereby amplifying inflammation in a positive feedback loop.
Dr. Rosenberg uses whole body radiation prior to the adoptive cell therapy. He has seen higher responses.
The Immune System needs three Signals:
The role of the CD8+ T cells is to monitor all the cells of the body, ready to destroy any that express foreign antigen fragments in their class I molecules.
Three major events must occur to Activate CD8+ T cell mediated response against melanoma.
First, the T-cell receptor (TRC) must be triggered by a (or multiple) self antigen–derived peptide MHC class I complex12. This event depends entirely on appropriate antigen presentation, which is most efficiently provided by mature dendritic cells. Once properly activated, may serve as tumor-specific effector T cells.
Second, simultaneously with T-cell receptor triggering, a distinct second costimulatory signal must be delivered, mediated by IL-2, B7-1, or B7-2, which engage IL-2 receptors and CD28 on the surface of the T cell, respectively . A source of these cofactors for effective CD8+ T-cell stimulation can be provided by CD4+ T cells that release critical amounts of IL-2, or by mature dendritic cells that display an increased level of B7-1/B7-2 costimulatory molecules on their cell surfaces.
Third, inflammatory cytokines, including IL-1, IL-6, IL-12, and IFN-γ provide a third signal that acts directly on T cells, referred to as the “danger signal”. This signal was found to optimally activate TH1 differentiation and lead to clonal expansion of T cells.
Best Regards,
Jimmy B
-
- You must be logged in to reply to this topic.